Why
BESREMi?
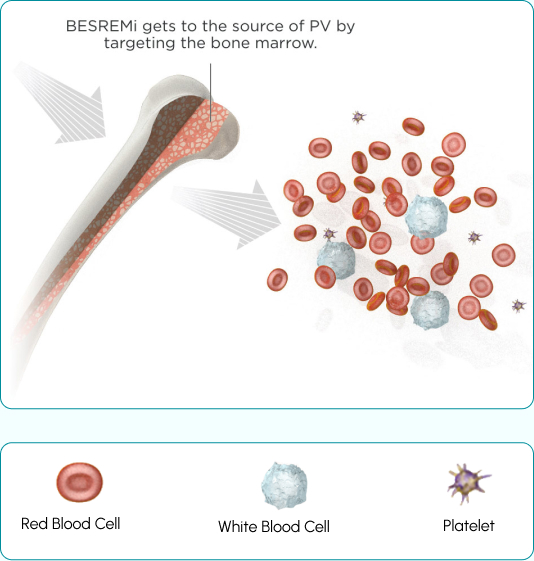
BESREMi is the only FDA-approved treatment indicated for PV that targets the bone marrow, helping to control blood cell counts. That’s what makes BESREMi different—it addresses the cause of PV.

BESREMi targets PV at the source of the disease
Now is the time: wherever you are in your journey with polycythemia vera (PV), it’s important that you understand your treatment options. Consider talking with your doctor about a treatment that targets PV at its source.
BESREMi is the only FDA-approved treatment indicated for PV that targets the bone marrow, helping to control blood cell counts. That’s what makes BESREMi different—it addresses the cause of PV.
BESREMi is not chemotherapy, it’s an innovative, long-acting interferon. This interferon is used as an immunotherapy that you take once every 2 weeks to treat PV. After maintaining stable blood levels for 1 year, you may be able to take BESREMi once every 4 weeks.
Ropeginterferon alfa-2b-njft (BESREMi) is a recommended treatment for certain patients with PV1
The National Comprehensive Cancer Network® (NCCN®) is a not-for-profit alliance of 33 leading cancer centers devoted to patient care, research, and education. The alliance creates national guidelines for recommended cancer treatment, called the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®).
The NCCN Guidelines® are:
- Designed to support decision-making
- A well-respected resource for doctors around the world
- Based on the latest data and clinical evidence
- Continuously updated and revised to reflect new information
These guidelines recommend ropeginterferon alfa-2b-njft (BESREMi) as a preferred first-line cytoreductive treatment option for certain patients with PV.1
BESREMi was proven to help a broad range of people living with PV
One study looked at the efficacy, dosing, and safety of BESREMi in adults diagnosed with PV.
People were included regardless of:
- History of cardiovascular events
- Prior treatment with hydroxyurea (HU), a type of chemotherapy
Of the people who used BESREMi in the clinical study:
- All were 35 to 82 years of age
- 33% had been treated with HU
- 22% had a previous thrombotic event
BESREMi showed meaningful outcomes
Complete Hematologic Response
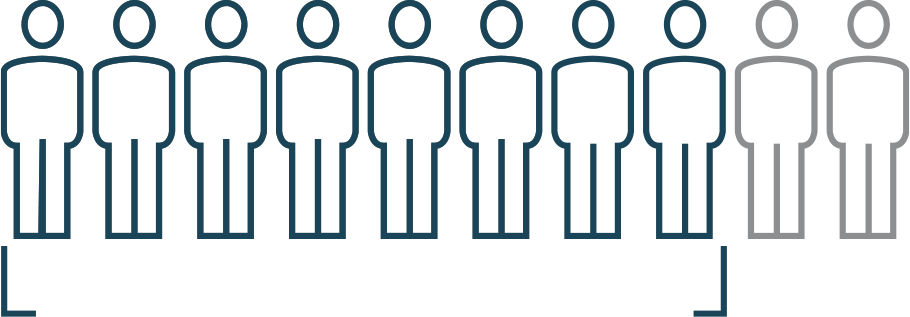
8 out of 10 achieved complete hematologic response
- Blood cell counts (red blood cells, white blood cells, and platelets) returned to a normal level
- No phlebotomy in the past 2 months
Comprehensive Disease Control
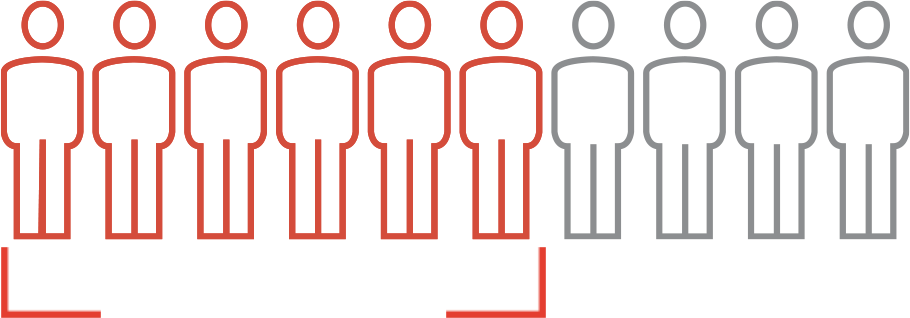
6 out of 10 achieved comprehensive disease control
- Blood cell counts returned to a normal level
- No phlebotomy in the past 2 months
- Normal spleen size
- No thromboembolic events
BESREMi displayed PV control over the long term
Complete hematologic response (CHR) was observed over a 7.5-year clinical trial.
Another study looked at the efficacy and safety of BESREMi in adults with PV over a period of 6 years.
People were included if they:
- Completed an initial study and benefited from BESREMi
- Had no history or less than 3 years of treatment with HU
Of the people who used BESREMi in the study:
- 22% had experienced a previous thrombotic event
- All were between 49 and 66 years old
- 32% had been treated with HU
- 22% had experienced a previous thrombotic event
BESREMi provided years without any phlebotomies
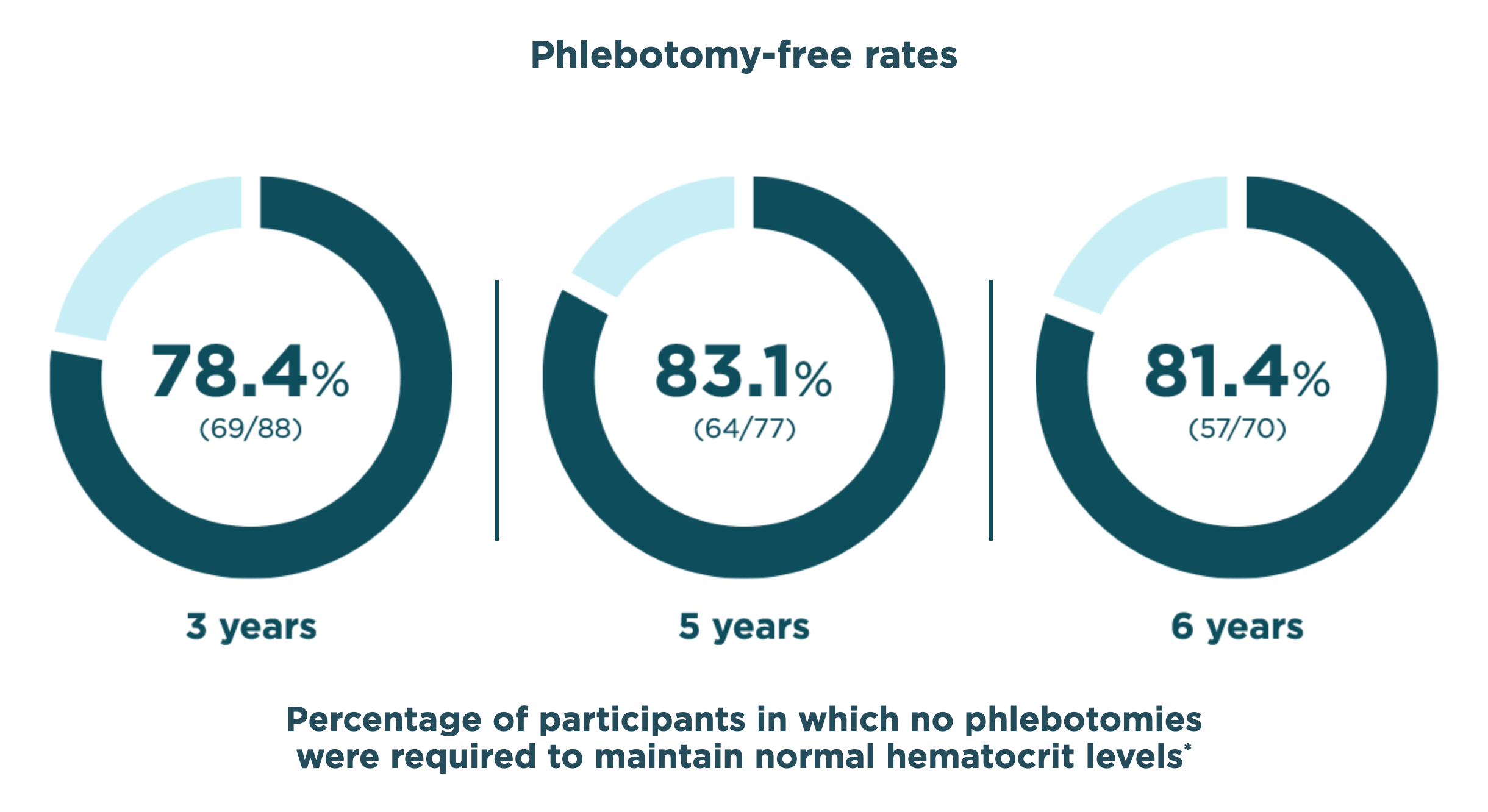
*Among those with available data for each treatment year.
Taking charge of my treatment: Susan’s story
Research and education may help you feel informed and empowered when exploring PV treatments. Watch Susan share how shaping her treatment plan with her doctor helped her discover BESREMi, which she chose as she continued her PV journey.
Watch more real stories about people using BESREMi →
How to get started with BESREMi
BESREMi is an at-home treatment that you administer
once every 2 weeks. Find out how to use BESREMi.
Possible side effects proven to lessen over time
Since BESREMi is an immunotherapy, it triggers your immune system to start working, which may make you more likely to experience some side effects. In clinical trials for BESREMi, some of these lessened over time.
IMPORTANT SAFETY INFORMATION
Reference: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myeloproliferative Neoplasms V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed February 25, 2025. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Read this Important Safety Information carefully. It explains the serious risks of BESREMi and how to take it safely. Talk to your healthcare provider if you have any questions.
What is the most important information I should know about BESREMi?
BESREMi can cause serious side effects, including conditions that may cause death or may worsen certain serious diseases you may already have. If symptoms get worse, or become severe and continue, your healthcare provider may tell you to stop taking BESREMi permanently. These symptoms may go away in some people after they stop taking BESREMi.
Mental health problems, including suicide
BESREMi may cause mood or behavior problems that can get worse during treatment or after your last dose, including:
- Irritability (getting upset easily)
- Restlessness and agitation
- Confusion
- Depression (feeling low, hopeless, or bad about yourself)
- Unusually grand ideas
- Acting aggressive or impulsively
- Thoughts of hurting yourself or others, or thoughts of suicide
If you develop any of these symptoms, call your healthcare provider immediately.
New or worsening autoimmune problems
BESREMi may cause your immune system to attack healthy cells, leading to conditions such as thyroid disease, increased blood sugar (hyperglycemia), or type 1 diabetes. Call your healthcare provider if you experience:
- Extreme tiredness
- Frequent urination
- Excessive thirst
Heart problems
BESREMi may cause heart problems, including:
- Cardiomyopathy (heart muscle disease)
- Heart attack
- Irregular heartbeat (atrial fibrillation)
- Decreased blood flow to the heart
You should not take BESREMi if you have:
- Uncontrolled high blood pressure
- Congestive heart failure
- A serious abnormal heart rhythm
- Narrowing of the arteries to your heart
- Certain types of chest pain (angina)
- A recent stroke or heart attack
Who should not take BESREMi?
Do not take BESREMi if you:
- Have or had severe mental health problems, especially depression, suicidal thoughts, or attempted suicide
- Have or had a serious or untreated autoimmune disease
- Are allergic to interferon or any ingredient in BESREMi (symptoms may include itching, swelling, trouble breathing, dizziness, or chest pain)
- Have certain types of liver disease
- Have had a transplant and take immune-suppressing medication
Before using BESREMi, tell your healthcare provider about all of your medical conditions, including if you have any of the following:
Do not take BESREMi if you:
- A mental illness
- Type 1 diabetes
- Heart or bleeding problems
- Problems with your immune system
- Hepatitis B or HIV infection
- Kidney or liver problems
- Are pregnant or planning to become pregnant
- BESREMi may harm your unborn baby. Use effective birth control during treatment and for at least 8 weeks after your last dose
- BESREMi may affect your menstrual cycle and could stop your periods
- Do not breastfeed while taking BESREMi
What are the possible side effects of BESREMi? Serious side effects include:
- Low blood cell counts: taking BESREMi can lead to infections, anemia, or bleeding problems. Call your healthcare provider right away if you develop weakness and tiredness, bruising easily, nose bleeds often, fever, chills, burning and painful urination, urinating often, or coughing up yellow or pink mucus (phlegm)
- Serious allergic reactions: Get medical help right away if you get any of the following symptoms: skin rash or hives; itching; swelling of the face, eyes, lips, tongue, or throat; trouble breathing; chest pain; or feeling faint
- Eye problems: BESREMi can cause severe eye problems with your retinas that can lead to vision loss or blindness. You should have an eye exam before and during treatment with BESREMi if you have diabetes or high blood pressure and also have retinal problems
- Liver problems: BESREMi can cause increases in liver enzymes and liver damage. Your healthcare provider should do blood tests to monitor your liver enzymes and liver function before you start and during treatment with BESREMi
- Kidney problems: Your healthcare provider will do blood tests to check your kidney function before starting and during treatment with BESREMi. Tell your healthcare provider right away if you develop any symptoms of a kidney problem, including: changes in the amount or color of your urine, swelling in your ankles, blood in your urine, or loss of appetite
- Tooth and gum problems: BESREMi can cause tooth loss and/or dry mouth. It is important for you to brush your teeth well, two times each day and have regular dental examinations during treatment with BESREMi
- Skin reactions: BESREMi may cause reaction such as rash, itching, and hair loss
- Increased triglycerides: You may require blood tests to monitor levels
The most common side effects of BESREMi include:
- Flu-like symptoms, including tiredness, weakness, fever, chills, muscle aches, and joint pain
- Itching
- Sore throat
These are not all of the possible side effects of BESREMi.
Call your doctor for medical advice about side effects. You may report side effects to PharmaEssentia at 1-800-999-2449 or FDA at 1-800-FDA-1088.
Please see full Prescribing Information, including Boxed Warning and Medication Guide for BESREMi.